Battery ratings
Because batteries create electron flow in a
circuit by exchanging electrons in ionic chemical reactions,
and there is a limited number of molecules in any charged
battery available to react, there must be a limited amount
of total electrons that any battery can motivate through a
circuit before its energy reserves are exhausted. Battery
capacity could be measured in terms of total number of
electrons, but this would be a huge number. We could use the
unit of the coulomb (equal to 6.25 x 1018
electrons, or 6,250,000,000,000,000,000 electrons) to make
the quantities more practical to work with, but instead a
new unit, the amp-hour, was made for this purpose.
Since 1 amp is actually a flow rate of 1 coulomb of
electrons per second, and there are 3600 seconds in an hour,
we can state a direct proportion between coulombs and
amp-hours: 1 amp-hour = 3600 coulombs. Why make up a new
unit when an old would have done just fine? To make your
lives as students and technicians more difficult, of course!
A battery with a capacity of 1 amp-hour
should be able to continuously supply a current of 1 amp to
a load for exactly 1 hour, or 2 amps for 1/2 hour, or 1/3
amp for 3 hours, etc., before becoming completely
discharged. In an ideal battery, this relationship between
continuous current and discharge time is stable and
absolute, but real batteries don't behave exactly as this
simple linear formula would indicate. Therefore, when
amp-hour capacity is given for a battery, it is specified at
either a given current, given time, or assumed to be rated
for a time period of 8 hours (if no limiting factor is
given).
For example, an average automotive battery
might have a capacity of about 70 amp-hours, specified at a
current of 3.5 amps. This means that the amount of time this
battery could continuously supply a current of 3.5 amps to a
load would be 20 hours (70 amp-hours / 3.5 amps). But let's
suppose that a lower-resistance load were connected to that
battery, drawing 70 amps continuously. Our amp-hour equation
tells us that the battery should hold out for exactly 1 hour
(70 amp-hours / 70 amps), but this might not be true in real
life. With higher currents, the battery will dissipate more
heat across its internal resistance, which has the effect of
altering the chemical reactions taking place within. Chances
are, the battery would fully discharge some time before
the calculated time of 1 hour under this greater load.
Conversely, if a very light load (1 mA) were
to be connected to the battery, our equation would tell us
that the battery should provide power for 70,000 hours, or
just under 8 years (70 amp-hours / 1 milliamp), but the odds
are that much of the chemical energy in a real battery would
have been drained due to other factors (evaporation of
electrolyte, deterioration of electrodes, leakage current
within battery) long before 8 years had elapsed. Therefore,
we must take the amp-hour relationship as being an ideal
approximation of battery life, the amp-hour rating trusted
only near the specified current or timespan given by the
manufacturer. Some manufacturers will provide amp-hour
derating factors specifying reductions in total capacity at
different levels of current and/or temperature.
For secondary cells, the amp-hour rating
provides a rule for necessary charging time at any given
level of charge current. For example, the 70 amp-hour
automotive battery in the previous example should take 10
hours to charge from a fully-discharged state at a constant
charging current of 7 amps (70 amp-hours / 7 amps).
Approximate amp-hour capacities of some
common batteries are given here:
-
Typical automotive battery: 70 amp-hours @
3.5 A (secondary cell)
-
D-size carbon-zinc battery: 4.5 amp-hours
@ 100 mA (primary cell)
-
9 volt carbon-zinc battery: 400
milliamp-hours @ 8 mA (primary cell)
As a battery discharges, not only does it
diminish its internal store of energy, but its internal
resistance also increases (as the electrolyte becomes less
and less conductive), and its open-circuit cell voltage
decreases (as the chemicals become more and more dilute).
The most deceptive change that a discharging battery
exhibits is increased resistance. The best check for a
battery's condition is a voltage measurement under load,
while the battery is supplying a substantial current through
a circuit. Otherwise, a simple voltmeter check across the
terminals may falsely indicate a healthy battery (adequate
voltage) even though the internal resistance has increased
considerably. What constitutes a "substantial current" is
determined by the battery's design parameters. A voltmeter
check revealing too low of a voltage, of course, would
positively indicate a discharged battery:
Fully charged battery:
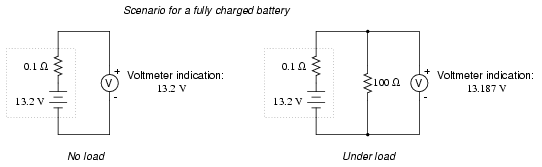
Now, if the battery discharges a bit . . .
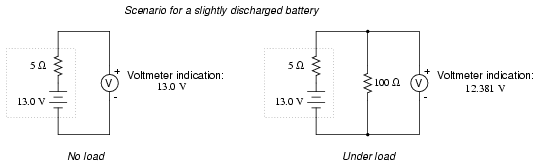
. . . and discharges a bit further . . .
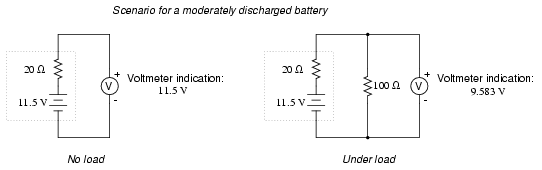
. . . and a bit further until it's dead.
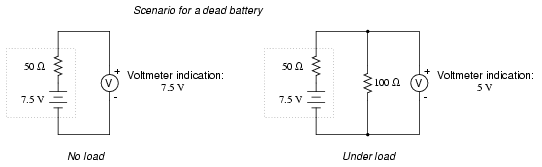
Notice how much better the battery's true
condition is revealed when its voltage is checked under load
as opposed to without a load. Does this mean that it's
pointless to check a battery with just a voltmeter (no
load)? Well, no. If a simple voltmeter check reveals only
7.5 volts for a 13.2 volt battery, then you know without a
doubt that it's dead. However, if the voltmeter were to
indicate 12.5 volts, it may be near full charge or somewhat
depleted -- you couldn't tell without a load check. Bear in
mind also that the resistance used to place a battery under
load must be rated for the amount of power expected to be
dissipated. For checking large batteries such as an
automobile (12 volt nominal) lead-acid battery, this may
mean a resistor with a power rating of several hundred
watts.
-
REVIEW:
-
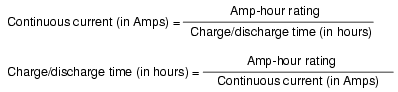
The amp-hour is a unit of battery
energy capacity, equal to the amount of continuous current
multiplied by the discharge time, that a battery can
supply before exhausting its internal store of chemical
energy.
-
-
An amp-hour battery rating is only an
approximation of the battery's charge capacity, and should
be trusted only at the current level or time specified by
the manufacturer. Such a rating cannot be extrapolated for
very high currents or very long times with any accuracy.
-
Discharged batteries lose voltage and
increase in resistance. The best check for a dead battery
is a voltage test under load.
|