Electron activity in
chemical reactions
So far in our discussions on electricity and
electric circuits, we have not discussed in any detail how
batteries function. Rather, we have simply assumed that they
produce constant voltage through some sort of mysterious
process. Here, we will explore that process to some degree
and cover some of the practical considerations involved with
real batteries and their use in power systems.
In the first chapter of this book, the
concept of an atom was discussed, as being the basic
building-block of all material objects. Atoms, in turn,
however, are composed of even smaller pieces of matter
called particles. Electrons, protons, and neutrons
are the basic types of particles found in atoms. Each of
these particle types plays a distinct role in the behavior
of an atom. While electrical activity involves the motion of
electrons, the chemical identity of an atom (which largely
determines how conductive the material will be) is
determined by the number of protons in the nucleus (center).
The protons in an atom's nucleus are
extremely difficult to dislodge, and so the chemical
identity of any atom is very stable. One of the goals of the
ancient alchemists (to turn lead into gold) was foiled by
this sub-atomic stability. All efforts to alter this
property of an atom by means of heat. light, or friction
were met with failure. The electrons of an atom, however,
are much more easily dislodged. As we have already seen,
friction is one way in which electrons can be transferred
from one atom to another (glass and silk, wax and wool), and
so is heat (generating voltage by heating a junction of
dissimilar metals, as in the case of thermocouples).
Electrons can do much more than just move
around and between atoms: they can also serve to link
different atoms together. This linking of atoms by electrons
is called a chemical bond. A crude (and simplified)
representation of such a bond between two atoms might look
like this:
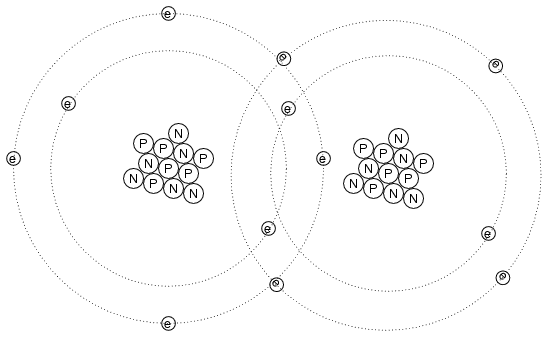
There are several types of chemical bonds,
the one shown above being representative of a covalent
bond, where electrons are shared between atoms. Because
chemical bonds are based on links formed by electrons, these
bonds are only as strong as the immobility of the electrons
forming them. That is to say, chemical bonds can be created
or broken by the same forces that force electrons to move:
heat, light, friction, etc.
When atoms are joined by chemical bonds,
they form materials with unique properties known as
molecules. The dual-atom picture shown above is an
example of a simple molecule formed by two atoms of the same
type. Most molecules are unions of different types of atoms.
Even molecules formed by atoms of the same type can have
radically different physical properties. Take the element
carbon, for instance: in one form, graphite, carbon
atoms link together to form flat "plates" which slide
against one another very easily, giving graphite its natural
lubricating properties. In another form, diamond, the
same carbon atoms link together in a different
configuration, this time in the shapes of interlocking
pyramids, forming a material of exceeding hardness. In yet
another form, Fullerene, dozens of carbon atoms form
each molecule, which looks something like a soccer ball.
Fullerene molecules are very fragile and lightweight. The
airy soot formed by excessively rich combustion of acetylene
gas (as in the initial ignition of an oxy-acetylene
welding/cutting torch) is composed of many tiny Fullerene
molecules.
When alchemists succeeded in changing the
properties of a substance by heat, light, friction, or
mixture with other substances, they were really observing
changes in the types of molecules formed by atoms breaking
and forming bonds with other atoms. Chemistry is the modern
counterpart to alchemy, and concerns itself primarily with
the properties of these chemical bonds and the reactions
associated with them.
A type of chemical bond of particular
interest to our study of batteries is the so-called ionic
bond, and it differs from the covalent bond in that
one atom of the molecule possesses an excess of electrons
while another atom lacks electrons, the bonds between them
being a result of the electrostatic attraction between the
two unlike charges. Consequently, ionic bonds, when broken
or formed, result in electrons moving from one place to
another. This motion of electrons in ionic bonding can be
harnessed to generate an electric current. A device
constructed to do just this is called a voltaic cell,
or cell for short, usually consisting of two metal
electrodes immersed in a chemical mixture (called an
electrolyte) designed to facilitate a chemical reaction:
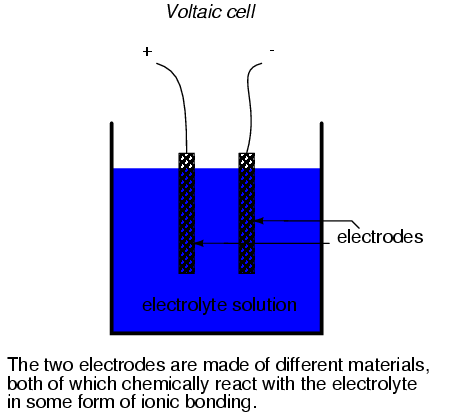
In the common "lead-acid" cell (the kind
commonly used in automobiles), the negative electrode is
made of lead (Pb) and the positive is made of lead peroxide
(Pb02), both metallic substances. The electrolyte
solution is a dilute sulfuric acid (H2SO4
+ H2O). If the electrodes of the cell are
connected to an external circuit, such that electrons have a
place to flow from one to the other, negatively charged
oxygen ions (O) from the positive electrode (PbO2)
will ionically bond with positively charged hydrogen ions
(H) to form molecules water (H2O). This creates a
deficiency of electrons in the lead peroxide (PbO2)
electrode, giving it a positive electrical charge. The
sulfate ions (SO4) left over from the
disassociation of the hydrogen ions (H) from the sulfuric
acid (H2SO4) will join with the lead (Pb)
in each electrode to form lead sulfate (PbSO4):
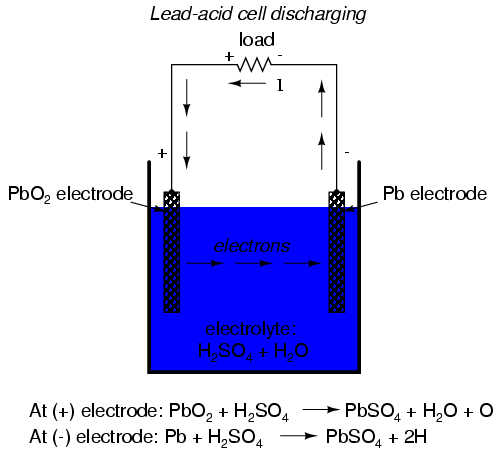
This process of the cell providing
electrical energy to supply a load is called discharging,
since it is depleting its internal chemical reserves.
Theoretically, after all of the sulfuric acid has been
exhausted, the result will be two electrodes of lead sulfate
(PbSO4) and an electrolyte solution of pure water
(H2O), leaving no more capacity for additional
ionic bonding. In this state, the cell is said to be
fully discharged. In a lead-acid cell, the state of
charge can be determined by an analysis of acid strength.
This is easily accomplished with a device called a
hydrometer, which measures the specific gravity
(density) of the electrolyte. Sulfuric acid is denser than
water, so the greater the charge of a cell, the greater the
acid concentration, and thus a denser electrolyte solution.
There is no single chemical reaction
representative of all voltaic cells, so any detailed
discussion of chemistry is bound to have limited
application. The important thing to understand is that
electrons are motivated to and/or from the cell's electrodes
via ionic reactions between the electrode molecules and the
electrolyte molecules. The reaction is enabled when there is
an external path for electric current, and ceases when that
path is broken.
Being that the motivation for electrons to
move through a cell is chemical in nature, the amount of
voltage (electromotive force) generated by any cell will be
specific to the particular chemical reaction for that cell
type. For instance, the lead-acid cell just described has a
nominal voltage of 2.2 volts per cell, based on a fully
"charged" cell (acid concentration strong) in good physical
condition. There are other types of cells with different
specific voltage outputs. The Edison cell, for
example, with a positive electrode made of nickel oxide, a
negative electrode made of iron, and an electrolyte solution
of potassium hydroxide (a caustic, not acid, substance)
generates a nominal voltage of only 1.2 volts, due to the
specific differences in chemical reaction with those
electrode and electrolyte substances.
The chemical reactions of some types of
cells can be reversed by forcing electric current backwards
through the cell (in the negative electrode and
out the positive electrode). This process is called
charging. Any such (rechargeable) cell is called a
secondary cell. A cell whose chemistry cannot be
reversed by a reverse current is called a primary cell.
When a lead-acid cell is charged by an
external current source, the chemical reactions experienced
during discharge are reversed:

-
REVIEW:
-
Atoms bound together by electrons are
called molecules.
-
Ionic bonds are molecular unions
formed when an electron-deficient atom (a positive ion)
joins with an electron-excessive atom (a negative ion).
-
Chemical reactions involving ionic bonds
result in the transfer of electrons between atoms. This
transfer can be harnessed to form an electric current.
-
A cell is a device constructed to
harness such chemical reactions to generate electric
current.
-
A cell is said to be discharged
when its internal chemical reserves have been depleted
through use.
-
A secondary cell's chemistry can be
reversed (recharged) by forcing current backwards through
it.
-
A primary cell cannot be
practically recharged.
-
Lead-acid cell charge can be assessed with
an instrument called a hydrometer, which measures
the density of the electrolyte liquid. The denser the
electrolyte, the stronger the acid concentration, and the
greater charge state of the cell.
|