Practical
considerations
When connecting batteries together to form
larger "banks" (a battery of batteries?), the
constituent batteries must be matched to each other so as to
not cause problems. First we will consider connecting
batteries in series for greater voltage:
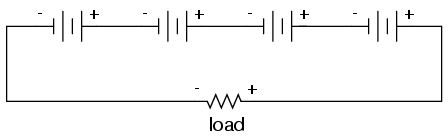
We know that the current is equal at all
points in a series circuit, so whatever amount of current
there is in any one of the series-connected batteries must
be the same for all the others as well. For this reason,
each battery must have the same amp-hour rating, or else
some of the batteries will become depleted sooner than
others, compromising the capacity of the whole bank.
Please note that the total amp-hour capacity of this series
battery bank is not affected by the number of batteries.
Next, we will consider connecting batteries
in parallel for greater current capacity (lower internal
resistance), or greater amp-hour capacity:
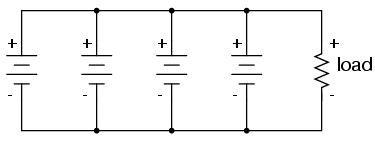
We know that the voltage is equal across all
branches of a parallel circuit, so we must be sure that
these batteries are of equal voltage. If not, we will have
relatively large currents circulating from one battery
through another, the higher-voltage batteries overpowering
the lower-voltage batteries. This is not good.
On this same theme, we must be sure that any
overcurrent protection (circuit breakers or fuses) are
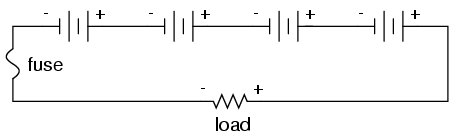
installed in such a way as to be effective. For our series
battery bank, one fuse will suffice to protect the wiring
from excessive current, since any break in a series circuit
stops current through all parts of the circuit:
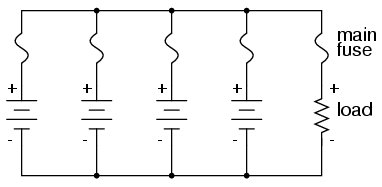
With a parallel battery bank, one fuse is
adequate for protecting the wiring against load overcurrent
(between the parallel-connected batteries and the load), but
we have other concerns to protect against as well. Batteries
have been known to internally short-circuit, due to
electrode separator failure, causing a problem not unlike
that where batteries of unequal voltage are connected in
parallel: the good batteries will overpower the failed
(lower voltage) battery, causing relatively large currents
within the batteries' connecting wires. To guard against
this eventuality, we should protect each and every battery
against overcurrent with individual battery fuses, in
addition to the load fuse:
When dealing with secondary-cell batteries,
particular attention must be paid to the method and timing
of charging. Different types and construction of batteries
have different charging needs, and the manufacturer's
recommendations are probably the best guide to follow when
designing or maintaining a system. Two distinct concerns of
battery charging are cycling and overcharging.
Cycling refers to the process of charging a battery to a
"full" condition and then discharging it to a lower state.
All batteries have a finite (limited) cycle life, and the
allowable "depth" of cycle (how far it should be discharged
at any time) varies from design to design. Overcharging is
the condition where current continues to be forced backwards
through a secondary cell beyond the point where the cell has
reached full charge. With lead-acid cells in particular,
overcharging leads to electrolysis of the water ("boiling"
the water out of the battery) and shortened life.
Any battery containing water in the
electrolyte is subject to the production of hydrogen gas due
to electrolysis. This is especially true for overcharged
lead-acid cells, but not exclusive to that type. Hydrogen is
an extremely flammable gas (especially in the presence of
free oxygen created by the same electrolysis process),
odorless and colorless. Such batteries pose an explosion
threat even under normal operating conditions, and must be
treated with respect. The author has been a firsthand
witness to a lead-acid battery explosion, where a spark
created by the removal of a battery charger (small DC power
supply) from an automotive battery ignited hydrogen gas
within the battery case, blowing the top off the battery and
splashing sulfuric acid everywhere. This occurred in a high
school automotive shop, no less. If it were not for all the
students nearby wearing safety glasses and buttoned-collar
overalls, significant injury could have occurred.
When connecting and disconnecting charging
equipment to a battery, always make the last connection (or
first disconnection) at a location away from the battery
itself (such as at a point on one of the battery cables, at
least a foot away from the battery), so that any resultant
spark has little or no chance of igniting hydrogen gas.
In large, permanently installed battery
banks, batteries are equipped with vent caps above each
cell, and hydrogen gas is vented outside of the battery room
through hoods immediately over the batteries. Hydrogen gas
is very light and rises quickly. The greatest danger is when
it is allowed to accumulate in an area, awaiting ignition.
More modern lead-acid battery designs are
sealed, using a catalyst to re-combine the electrolyzed
hydrogen and oxygen back into water, inside the battery case
itself. Adequate ventilation might still be a good idea,
just in case a battery were to develop a leak in the case.
-
REVIEW:
-
Connecting batteries in series increases
voltage, but does not increase overall amp-hour capacity.
-
All batteries in a series bank must
have the same amp-hour rating.
-
Connecting batteries in parallel increases
total current capacity by decreasing total resistance, and
it also increases overall amp-hour capacity.
-
All batteries in a parallel bank must
have the same voltage rating.
-
Batteries can be damaged by excessive
cycling and overcharging.
-
Water-based electrolyte batteries are
capable of generating explosive hydrogen gas, which must
not be allowed to accumulate in an area.
|